New Research on Classic Meds
Some drugs have been used for such a long time and so frequently that medicine can no longer be imagined without them – for example, aspirin, the antibiotic penicillin, or heparin
Feb 08, 2021
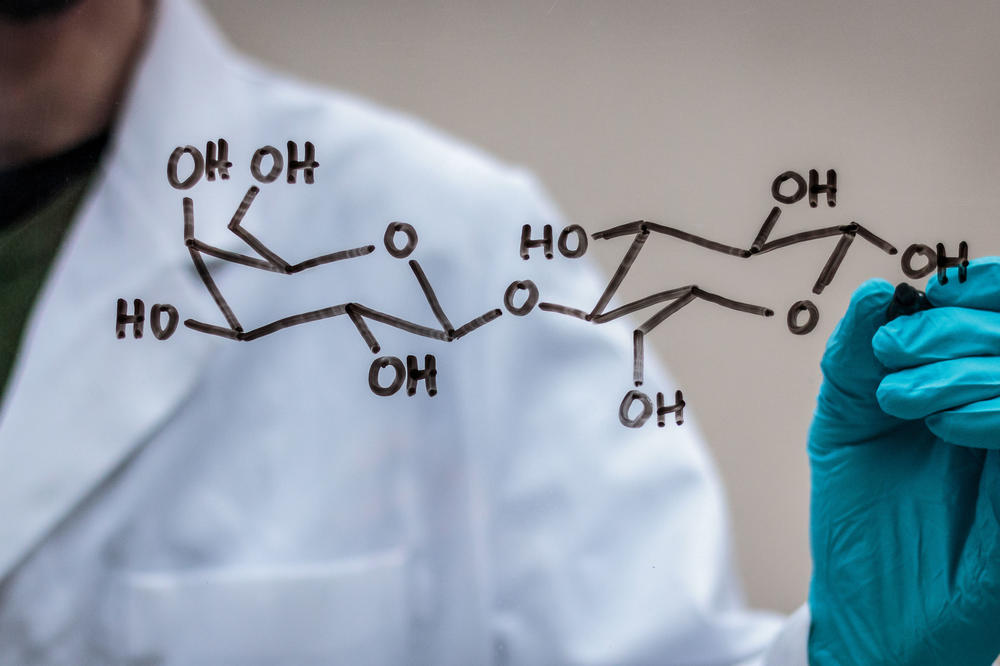
Tracking down the composition of heparin: Disaccharides – formerly called double sugars – are organic-chemical compounds from the group of carbohydrates.
Image Credit: Eike Mucha
The blood thinner heparin is injected into patients prophylactically whenever there is a risk of blood clots forming and blocking a vessel. In particular, this is intended to prevent thromboses and pulmonary embolisms in patients after an operation or in elderly, bedridden people. It is also used to treat blood-clotting disorders.
“Heparin was approved as a drug back in the 1940s, and it works really well. It is the drug of choice, the so-called gold standard in anticoagulation. By today’s standards, however, no drug agency in the world would approve it because basically nobody knows exactly what’s inside,” says Kevin Pagel, a professor of organic chemistry at Freie Universität Berlin. That is because heparin is not a standardized drug that consists of a single molecule, but a wild mixture of polysaccarides with variable chain lengths that occur in every animal organism.
Analyzing Sugar Chains in More Detail
A consortium of researchers at European universities now wants to analyze these sugar chains more closely as part of a Horizont 2020 project endowed with four million euros. Kevin Pagel’s group was granted 880,000 euros. Researchers at the University of Copenhagen, the University of Utrecht, Radboud University (in Nijmegen, the Netherlands), the Stockholm Karolinska Institute, and three companies are also involved.
The researchers intend to precisely clarify the structures of heparin. They want to set up a technology platform for purposes of quality control in order to be able to measure what is actually contained in a batch of raw heparin. In addition, they will explore possibilities for producing the blood thinner synthetically.
From the Intestinal Lining of Pigs
It is high time for that: heparin, or rather the heparins, are most efficiently obtained from the intestinal mucosa of pigs. China is the world’s largest producer of the raw product. More than ten tons are exported from there alone to Germany every year. Impurities in the raw heparin – through poor extraction and an illegally added surrogate – led to allergic shock reactions in many patients around the world in 2007/2008. In the USA alone, almost 800 people became seriously ill, and 80 died.
Kevin Pagel, a professor of organic chemistry, is researching the practical application of the anticoagulant heparin.
Image Credit: Eike Mucha
From a chemical point of view, heparins belong to the group of glycosaminoglycans. While other natural polysaccharides such as cellulose or chitin have an exactly regular structure, the only thing that is regular about heparins is that two different basic building blocks – a glucosamine and a sugar acid – alternate in the chain like two different types of pearls.
But there are two variants of each of the building blocks. And what’s more, the “pearls” are often equipped with small appendages. These are sulfate groups that can also be attached to different hydroxyl groups (-OH) on the sugar molecules. “It couldn’t be more heterogeneous,” says Kevin Pagel.
The sugar chains are formed on the membrane of certain cells, then gradually equipped with sulfate groups and at some point “cut off.” There can be just a few, but there can also be up to 100 or more sugars.
A Wind Tunnel for Molecules
The scientists in Stockholm are pursuing medical issues, and those in Copenhagen are carrying out gene modifications and part of the sequencing. The two Dutch groups are working on the artificial synthesis of the active ingredient. Pagel and his group are focusing on the structural analysis of smaller chain fragments.
Their work is based on the fact that heparins are highly negatively charged due to the many sulfate groups, which means that larger chains can therefore be easily broken down into smaller fragments in the mass spectrometer. However, these fragments can hardly be distinguished based on their mass because the basic structure is very similar. To get better results, Pagel therefore uses an ion mobility spectrometer behind the mass spectrometer.
“It works like a wind tunnel for molecules, a kind of chromatography in the gas phase,” he explains. Using smaller sugars, he was able to show in advance that two heparin fragments of the same size, which are sulfated differently, differ significantly in their three-dimensional structure. Depending on their size, shape, and charge, the ions fly through the measuring arrangement at different speeds. “It’s like a Ferrari and a Ford Transit: both have the same mass but a completely different drag.” But yes, the “engine” is equally strong in this case, he adds with a laugh. “Because that is the voltage that we apply to the wind tunnel.”
Huge Potential for Use of Heparin
First, Pagel and his colleagues disassembled, precisely measured, and reconstructed a heparin sequence made up of six sugar building blocks. In the meantime they also succeeded in analyzing a “chain of nine.” Pagel points out, “Six, eight, ten, or twelve are the sizes that are currently easy to handle. This means that the fragments still have sufficient overlap to be able to reconstruct the sequence in the chain afterward.”
The scientists have already analyzed an important sugar sequence via which heparin docks with certain proteins in the blood (so-called antithrombins) and thereby prevents blood clotting. However, it will take ten years before the structure of a long heparin made up of 100 sugar components can be fully analyzed.
“If everything goes well, that would be a huge step for the future use of heparins,” enthuses Pagel. He sees the primary application in the quality control of heparin preparations and the characterization of pharmaceutically more accessible heparin mimetics. For the longer term, he is also thinking of personalized medicine. “We could then determine the natural heparins in the blood of patients in order to perfectly tailor therapies to the individual.”
The project could also advance clinical diagnostics because sulfate groups at certain positions of the sugar molecules indicate inflammation in the body or tumors.
This text originally appeared in German on December 5, 2020, in the Tagesspiegel newspaper supplement published by Freie Universität.
Further Information
Professor Dr. Kevin Pagel, Freie Universität Berlin, Institute of Chemistry and Biochemistry, Email: kevin.pagel (at) fu-berlin.de